What Our Clients Have to Say
“Thank you for all your hard work and great communication. I know we are in good hands with your organization.”
– Suzie, Buyer | Gaming Industry
WHAT WE DO
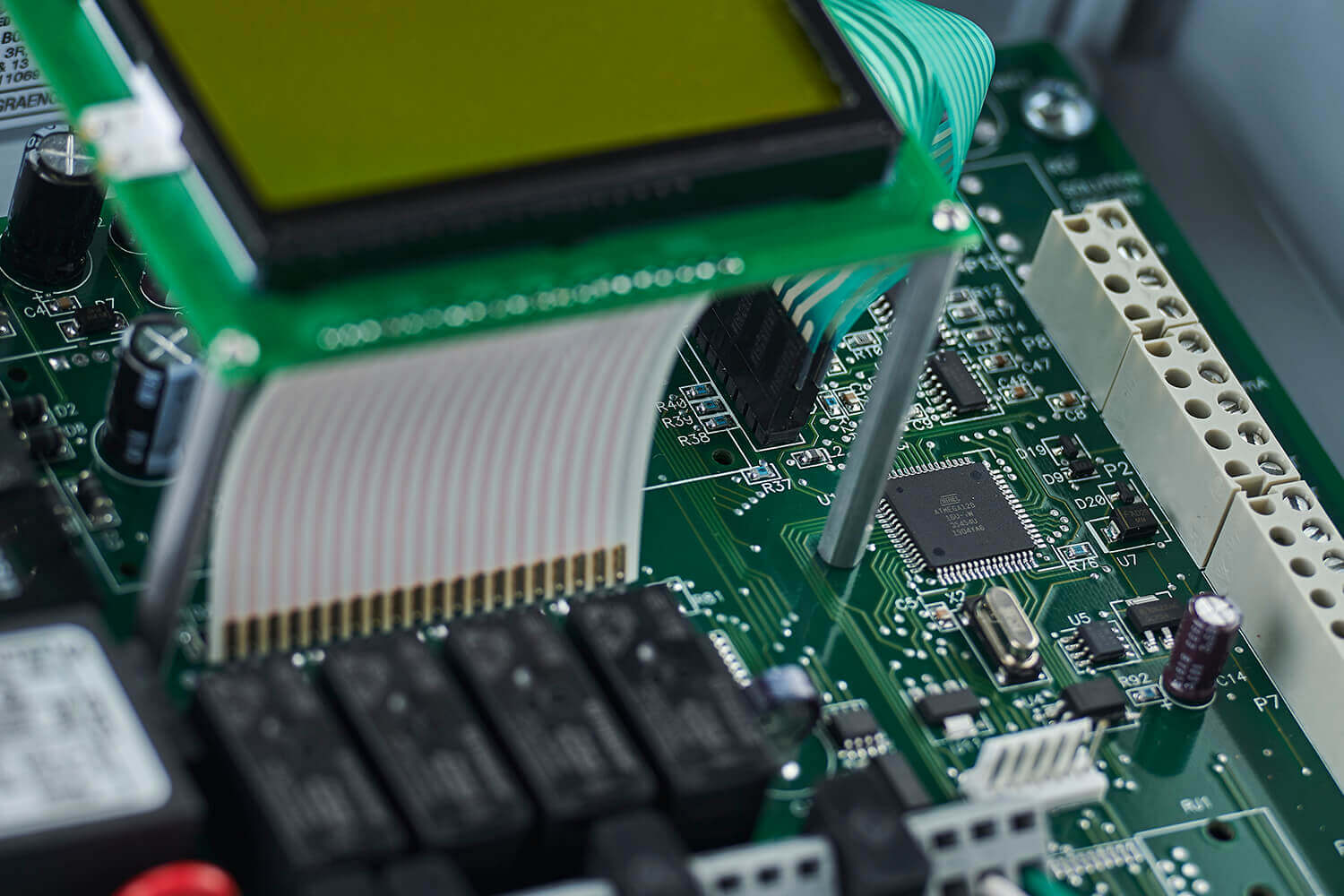
Since 1990, we have supplied specialized components to OEMs of medical equipment and medical and scientific instrumentation, from respirators to mass spectrometers. Our experienced technical resources and customized solutions provide the excellent quality and reliability required for lifesaving and life-improving products.
In addition to printed circuit board assembly (PCBA), we provide wire harness assembly, custom cable assemblies and electro-mechanical box builds, offering an integrated, one-stop solution. Your needs are our top priority. We strive to stay focused and agile as we help our customers manufacture innovative products used in clinical care or research and diagnostics.

In medical device development, early design optimization directly impacts manufacturability, quality, and regulatory readiness. As experienced medical electronics manufacturers, we work collaboratively with design and engineering teams to refine medical PCB assembly and medical electronic assembly designs for efficient production.
Our DFM support for medical electronics manufacturing includes:
By integrating manufacturability insights early, we help reduce production challenges, improve assembly performance, and support products built for demanding clinical environments.
CAPABILITIES
Assembly to your exact specifications, for intensive care or the dentist’s chair.

Robust cable assemblies for the most critical medical applications.

Expertise and proven processes deliver high-quality wire harnesses.
Sophisticated capabilities make us a trusted partner for customers in the medical field.

WHY US?

Your complex requirements for high-quality components used in medical and scientific products call for a trusted supply chain partner with sophisticated, integrated capabilities. Rigorous, reliable and responsive, we deliver quality and value through continuous improvement, personalized service and ISO 9001:2015 quality management.
Our printed circuit board assemblies, custom cable assemblies and wiring harnesses are built to your specifications for use in products such as critical care ventilators, bone densitometers, mass spectrometers, RO water purification equipment, and controls for power procedure tables, exam tables, and dental seating. Our customers include companies such as Getinge, Brewer, and Bruker.
“Thank you for all your hard work and great communication. I know we are in good hands with your organization.”
– Suzie, Buyer | Gaming Industry
“First of all, we would like to thank you and the entire staff at ETI who were involved to make sure the first round of boards got completed and delivered on-time. We are very delighted to say the boards are now being tested and working well.”
–Dino, Engineering Manager | Food Equipment Products
“Thank you so much for your help and extremely quick service! You really got us out of a jam, and we appreciate your understanding.”
– Bill, Product Manager | Tube & Pipe Cutting Equipment
We provide full medical PCB assembly, wire harnesses, custom cable assemblies, and electro-mechanical medical electronic assembly solutions tailored for OEM medical and scientific equipment.
Yes. We support everything from low-volume prototypes to high-volume production, adapting processes to meet quality and scalability needs typical in medical device supply chains.
We build assemblies using repeatable, controlled processes and inspections designed to meet the performance, uptime, and safety expectations of medical devices in clinical use.
Absolutely. We review engineering changes for manufacturability and help implement revisions without compromising schedule or quality as your product evolves.
Testing approaches may include visual inspection, automated optical inspection (AOI), functional testing, and other verification steps appropriate for the specific medical application.
Early engagement — ideally during design or prototyping — allows our team to provide valuable DFM guidance that reduces downstream manufacturing issues and speeds time-to-market.